Human Comprehensive Cancer Gene Mutation Test Kit
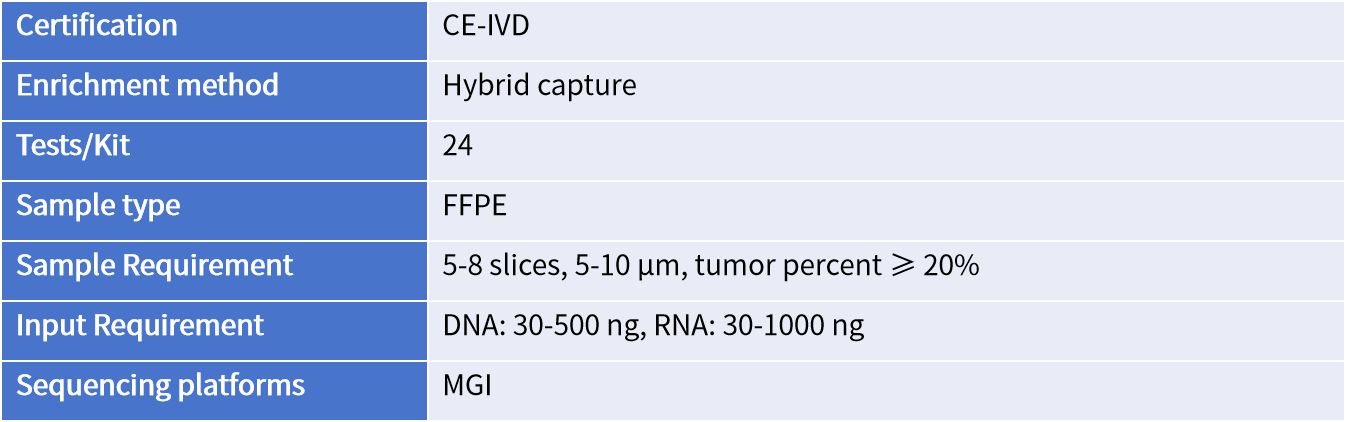
Genecast Comprehensive is a DNA+RNA based NGS assay with superb performance, comprehensive genomic profiles from FFPE sample. SNV, Indel, CNV, MSI and TMB are detected from FFPE DNA in 769 genes, while fusion and rearrangement are detected from FFPE RNA in 52 genes.
Genecast Comprehensive provides information of molecular typing, guidance on targeted, immunotherapeutic, and chemotherapeutic drugs, drug resistance insights, and data on hereditary oncogene mutations.
Key Features and Benefits
Comprehensive Coverage
Covers multiple types of genomic variants and biomarkers in 769 cancer-related genes for therapy selection, diagnosis, and prognosis in solid tumors.
High Accuracy
Analyzes SNV/Indel, CNV, MSI, and TMB from DNA, and fusion from RNA, with superior performance specifications.
Comprehensive Assessment of Immuno-Oncology (IO) Markers
DNA + RNA,covers more comprehensive biomarkers
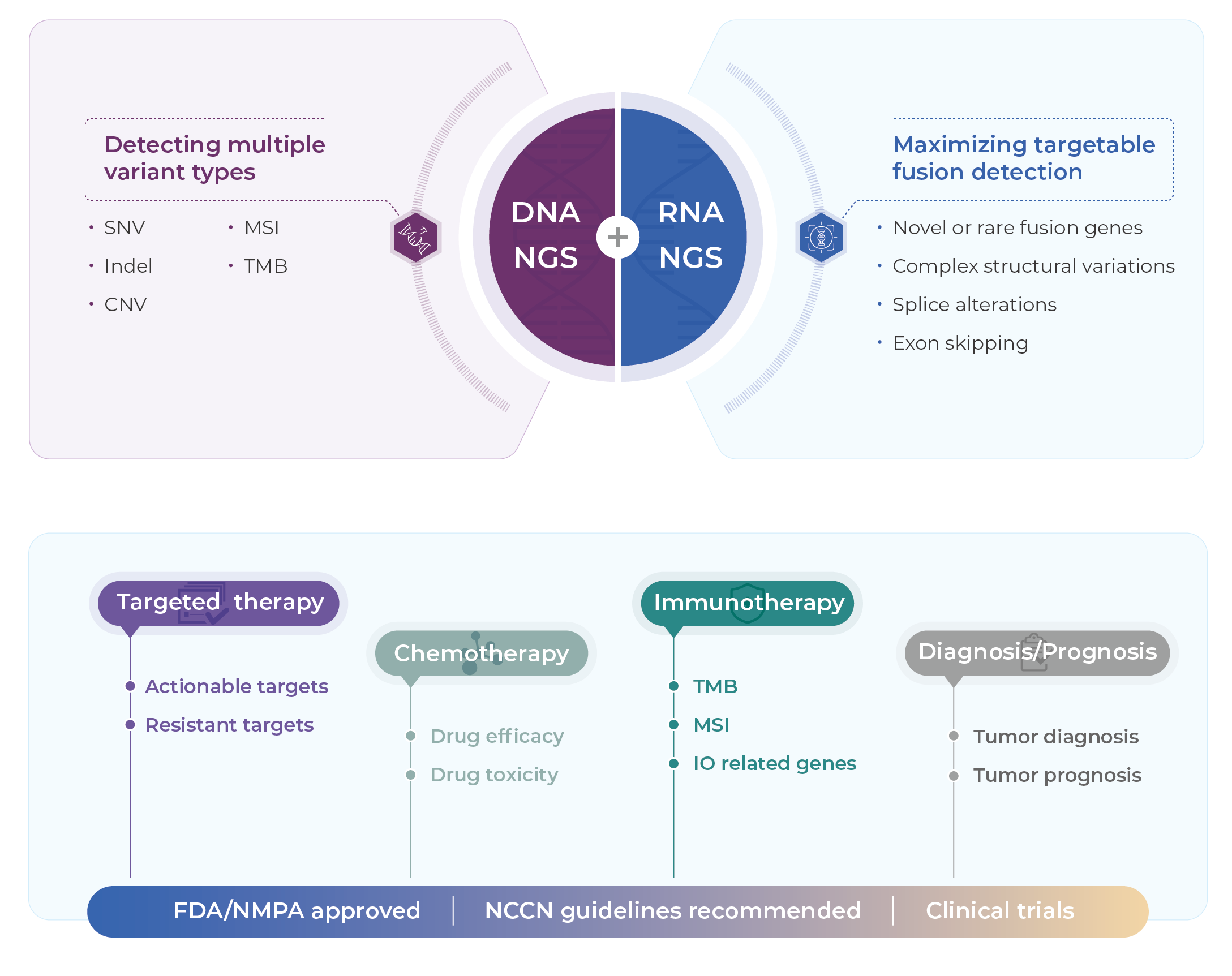
Specifications