Human ctDNA Focus Gene Mutation Test Kit
Genecast ctDNA Focus is a molecular diagnostic product for guiding medication specifically designed for patients of non-small cell lung cancer and colorectal cancer.
Genecast ctDNA Focus is a hybrid capture based targeted sequencing assay designed to evaluate somatic variants across multiple driver genes from blood samples. It provides comprehensive mutation data crucial for assessing sensitivity to targeted drugs and prognostic factors, including SNV, Indel, CNV, and fusion/rearrangement, MSI.
Key Features and Benefits
Profession Design
Grounded in extensive rigorous scientific validation, covering genes recommended by clinical guidelines of solid tumors
Comprehensive interpretation of multiple variations, providing professional and precise assistance for clinical decision-making
Comprehensive Coverage
Includes a broader range of genes related to targeted therapy
Provides extensive information on targeted therapy opportunities and treatment insights
Efficiency
Tests multiple genes and various mutation types simultaneously, with increased detection efficiency
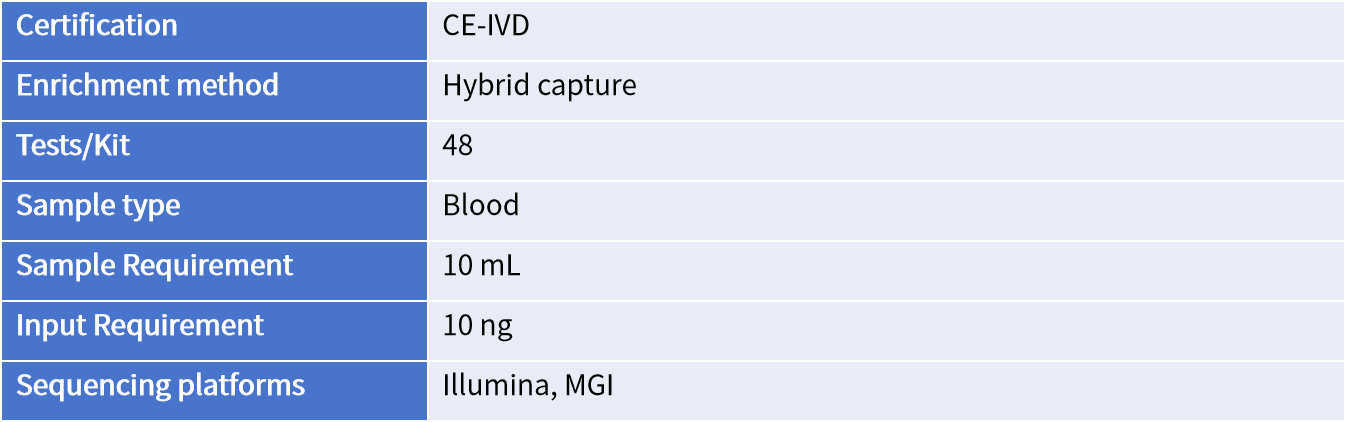
Gene List
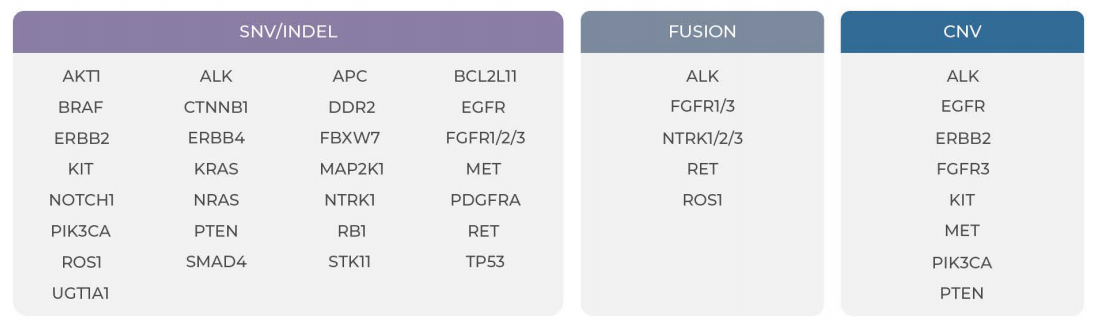
Specifications